What does Boiling Point at Elevation calculator do?
It is one of the tools created by MyCurrentElevation.org that helps you find the boiling point (Water) at your current altitude.
This tool relies on advanced geolocation technology, Google Maps, and other resources to provide you with real-time elevation data by just accessing your current location using your browser. You can manually enter your location and find out the boiling point of water at that elevation.
You can trust MyCurrentElevation.org’s boiling point calculator tool for accurate data. We believe our hours of hard work behind this tool will never let you down.
What is the Boiling Point?
Let’s start from the very start! What is boiling?
For starters, boiling is a physical process in which a liquid changes its state to gaseous form when it is heated. Some complex scientific phenomenon takes place at the molecular level. But, in easy language, we can say that heat or energy excites the molecule to move fast enough to change its state.
The temperature at which the boiling starts or the liquid starts changing its state is called Boiling Point.

At boiling point, the vapor pressure of the liquid equals the surrounding Atmospheric Pressure. That’s why, in different elevation, a liquid has different boiling points because atmospheric pressure changes as the height changes. With our Atmospheric Pressure at Elevation Calculator you can find out atmospheric pressure at your location.
In the next part, we will see how the boiling point of a liquid differs when put in a different region.
Change of Boiling Point with Elevation
Now, let us see how the boiling point of a liquid or water changes with the elevation at which the liquid is being heated.
In physical chemistry, the Clausius-Clapeyron equation can be used to understand how the pressure of a liquid’s vapor changes with temperature, which is especially useful in understanding boiling points.
And the simplified form of the Clausius-Clapeyron equation is –
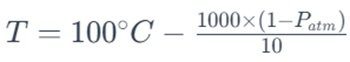
Here, P(atm) denotes Atmospheric Pressure. If you are interested you can check our Atmospheric Pressure at Elevation Calculator.

You see, the boiling point of liquid or water, in our case, is not directly dependent on the elevation. As per the equation, it is dependent only on atmospheric pressure. As you know, the value of atmospheric pressure decreases as we are higher in the earth’s atmosphere.
So, as the elevation increases, the boiling point of the liquid decreases. We can say that it is ‘easier’ to boil water at a hill station. Umm, that doesn’t sound correct. Let’s try again! It is easier to boil water at a hill station, but arranging the heat required to reach the boiling temperature might be a challenging task.
The values, boiling point, and elevation are inversely proportional to each other.
Boiling Point of Water at Elevation/ Altitude
Now you know how boiling point depends indirectly on the elevation at which the liquid is put. We will see how the value boiling point of water changes at different locations.

– Boiling point of Water at sea level
100 °C – The value of the boiling point of water we are familiar with is calculated at sea level. You will see a change of 0.5 °C when you change the elevation by 500 ft or around 152 meters. An increase in elevation results in a decrease in boiling point and vice versa.
– Boiling point of Water at Mount Everest
Earth’s highest point is Mount Everest, which has an elevation of 8,849 meters. The boiling point of water at Mount Everest is just 68 °C or 154.4 °F. But again, as we discussed earlier, managing sources to heat water at this temperature is not easy.
– Boiling point of Water at Vacuum
As per the simplified version of the Clausius-Clapeyron equation, the water can boil at any temperature above 0 °C. But, this is a completely theoritical value as perfect vacuum state cannot be acheived. But, in an environment where vacuum like conditions are created, water can even boil at room temperature.
– Impact on Cooking
In high-altitude areas, cooking takes longer because the lower boiling point of water means that foods boiled in water (like pasta or rice) will cook at a lower temperature, and that’s why it requires more time to become soft and edible.
– Impact on Human Physiology
At high altitudes, the lower boiling point of water can also affect human physiology. For instance, when climbing to high altitudes, the lower boiling point can impact how effectively our bodies can cool themselves through sweating.
